Cannabinoid Clinical Trial Pipeline Gains Momentum: 50+ Companies Lead the Charge in Pioneering New Treatments | DelveInsight
Cannabinoids are specific chemical compounds found in cannabis, such as tetrahydrocannabinol (THC) and cannabidiol (CBD). Research is increasingly supporting the efficacy of cannabinoids like CBD (cannabidiol) and THC (tetrahydrocannabinol) in treating a wide range of conditions, including mental health disorders, neurodegenerative diseases, and chronic inflammation.
New York, USA, Aug. 12, 2025 (GLOBE NEWSWIRE) -- Cannabinoid Clinical Trial Pipeline Gains Momentum: 50+ Companies Lead the Charge in Pioneering New Treatments | DelveInsight
Cannabinoids are specific chemical compounds found in cannabis, such as tetrahydrocannabinol (THC) and cannabidiol (CBD). Research is increasingly supporting the efficacy of cannabinoids like CBD (cannabidiol) and THC (tetrahydrocannabinol) in treating a wide range of conditions, including mental health disorders, neurodegenerative diseases, and chronic inflammation.
DelveInsight’s 'Cannabinoid Pipeline Insight 2025' report provides comprehensive global coverage of pipeline cannabinoid therapies in various stages of clinical development, major pharmaceutical companies are working to advance the pipeline space and future growth potential of the cannabinoid pipeline domain.
Key Takeaways from the Cannabinoid Pipeline Report
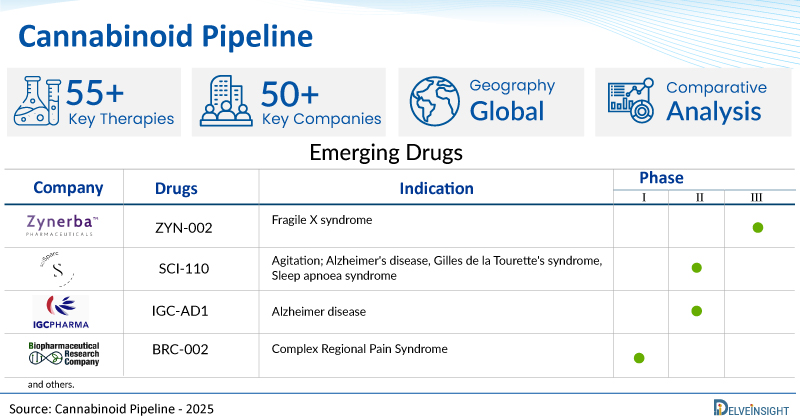
- DelveInsight’s cannabinoid pipeline report depicts a robust space with 50+ active players working to develop 55+ pipeline cannabinoid drugs.
- Key cannabinoid companies such as Zynerba Pharmaceuticals, Incannex Healthcare, IGC Pharma, INC., SciSparc, Biopharmaceutical Research Company, Polyrizon, SciSparc, Innocan Pharma Corporation, Apurano Pharmaceuticals, Artelo Biosciences, Inc., Octavian Therapeutics, Avata Biosciences, and others are evaluating new cannabinoid drugs to improve the treatment landscape.
- Promising pipeline cannabinoid therapies, such as ZYN-002, IHL-675A, IGC-AD1, SCI-110, BRC-002, SCI-160, Cannabidiol-loaded LPT, AP-707, ART27.13, OCT461201, AVAT 022, and others, are in different phases of cannabinoid clinical trials.
- In July 2025, MIRA Pharmaceuticals announced positive preclinical data demonstrating that Mira-55, the Company’s proprietary non-psychotropic marijuana analog, delivered morphine-comparable pain relief in a validated model of inflammatory pain-without causing local inflammation.
- In June 2025, InMed Pharmaceuticals announced new preclinical data demonstrating that INM-901 significantly reduces inflammation in ex vivo models of neuroinflammation, further supporting its potential as a therapeutic candidate in Alzheimer’s disease.
- In March 2025, Corbus Pharmaceuticals Holdings announced the dosing of the first subject in the single ascending dose / multiple ascending dose (SAD/MAD) portion of the Phase I trial of CRB-913 cannabinoid type-1 (CB1) receptor inverse agonist drug for the treatment of obesity. The study is being conducted in the United States under an open IND.
- In January 2025, IGC Pharma, Inc. announced that its ongoing Phase II trial for agitation in Alzheimer’s disease has been officially named CALMA (Calming Agitation in Alzheimer’s).
- In December 2024, Biopharmaceutical Research Company (BRC), announced that the US Food and Drug Administration (FDA) granted Orphan Drug Designation for the treatment of Complex Regional Pain Syndrome (CRPS) to BRC-002, which is being investigated in an investigator-initiated clinical Phase I trial.
- In September 2024, IGC Pharma, Inc. announced data that reinforces the therapeutic potential of IGC-AD1 as a disease-modifying treatment for Alzheimer's disease. The data highlights IGC-AD1's promising effects on tau tangles and spatial memory. These results build on earlier data demonstrating IGC-AD1's potential to reduce amyloid plaque.
Request a sample and discover the recent advances in cannabinoid drugs @ Cannabinoid Pipeline Report
The cannabinoid pipeline report provides detailed profiles of pipeline assets, a comparative analysis of clinical and non-clinical stage cannabinoid drugs, inactive and dormant assets, a comprehensive assessment of driving and restraining factors, and an assessment of opportunities and risks in the cannabinoid clinical trial landscape.
Cannabinoid Overview
Cannabinoids refer to a wide range of chemical substances that interact with the endocannabinoid system, a complex network of receptors and signaling molecules responsible for regulating vital bodily functions such as pain perception, mood, appetite, and immune system activity. These compounds can either be naturally sourced from the cannabis plant or created synthetically. The most extensively studied cannabinoids are tetrahydrocannabinol (THC), known for its mind-altering effects, and cannabidiol (CBD), recognized for its therapeutic benefits without causing intoxication. Cannabinoids are being explored for their potential in treating numerous health conditions, including epilepsy, chronic pain, inflammation, and nausea, driving significant scientific interest and drug development.
Cannabinoids are generally categorized into three primary types:
- Phytocannabinoids, which occur naturally in the cannabis plant and include well-known compounds like THC, CBD, and cannabigerol (CBG). These substances interact with cannabinoid receptors in the body to produce various physiological effects.
- Endocannabinoids, such as anandamide and 2-arachidonoylglycerol (2-AG), are molecules produced by the human body that help regulate essential functions like mood, appetite, and pain response.
- Synthetic cannabinoids are laboratory-engineered chemicals designed to replicate or modify the actions of natural cannabinoids. While they are used in research and therapeutic applications, some unauthorized synthetic variants have been linked to harmful side effects.
The cannabis plant itself contains an estimated 80 to 100 cannabinoid compounds, along with around 300 other non-cannabinoid substances. Among these, delta-9-tetrahydrocannabinol (THC) is the most recognized for producing cannabis’s psychoactive effects, while cannabidiol (CBD) is valued for its medicinal properties.
Therapeutically, cannabinoids have demonstrated the ability to influence the endocannabinoid system to help restore physiological balance. CBD, a non-psychoactive cannabinoid, has gained FDA approval for treating rare epilepsy forms like Lennox-Gastaut and Dravet syndromes through the drug Epidiolex. Additionally, it shows promise in managing conditions such as anxiety, inflammation, and neurodegenerative diseases. THC has proven effective for relieving chronic pain, reducing chemotherapy-related nausea and vomiting, and enhancing appetite in individuals with HIV/AIDS. Furthermore, a combination of THC and CBD is under investigation for treating multiple sclerosis and certain cancer-related pain.
Beyond THC and CBD, other cannabinoids such as cannabigerol (CBG) and cannabinol (CBN) are being studied for their therapeutic potential in conditions like glaucoma and insomnia. Synthetic cannabinoids, including FDA-approved medications like nabilone and dronabinol, provide standardized dosing options for managing nausea and appetite loss. Ongoing research continues to explore the potential of cannabinoids in treating autoimmune disorders, post-traumatic stress disorder (PTSD), and opioid addiction, highlighting their promise as safer alternatives or complementary therapies in modern medicine.
Find out more about cannabinoid drugs @ Cannabinoid Treatment
A snapshot of the Pipeline Cannabinoid Drugs mentioned in the report:
| Drugs | Company | Phase | Indication | RoA |
| ZYN-002 | Zynerba Pharmaceuticals | III | Fragile X syndrome | Transdermal |
| SCI-110 | SciSparc | II | Agitation; Alzheimer's disease, Gilles de la Tourette's syndrome, Sleep apnoea syndrome | Oral |
| IGC-AD1 | IGC Pharma, INC | II | Alzheimer disease | Oral |
| BRC-002 | Biopharmaceutical Research Company | I | Complex Regional Pain Syndrome | Oral |
| Cannabidiol-loaded LPT | Innocan Pharma Corporation | Preclinical | Epilepsy, Pain | Unspecified |
Learn more about the emerging cannabinoid therapies @ Cannabinoid Clinical Trials
Cannabinoid Therapeutics Assessment
The cannabinoid pipeline report proffers an integral view of the emerging cannabinoid therapies segmented by stage, product type, molecule type, route of administration, and mechanism of action.
Scope of the Cannabinoid Pipeline Report
- Coverage: Global
- Therapeutic Assessment By Product Type: Mono, Combination, Mono/Combination
- Therapeutic Assessment By Clinical Stages: Discovery, Pre-clinical, Phase I, Phase II, Phase III
- Therapeutics Assessment By Route of Administration: Intravenous, Subcutaneous, Oral, Intramuscular
- Therapeutics Assessment By Molecule Type: Monoclonal antibody, Small molecule, Peptide
- Key Cannabinoid Companies: Zynerba Pharmaceuticals, Incannex Healthcare, IGC Pharma, INC., SciSparc, Biopharmaceutical Research Company, Polyrizon, SciSparc, Innocan Pharma Corporation, Apurano Pharmaceuticals, Artelo Biosciences, Inc., Octavian Therapeutics, Avata Biosciences, and others.
- Key Cannabinoid Pipeline Therapies: ZYN-002, IHL-675A, IGC-AD1, SCI-110, BRC-002, SCI-160, Cannabidiol-loaded LPT, AP-707, ART27.13, OCT461201, AVAT 022, and others.
Dive deep into rich insights for new cannabinoid treatments, visit @ Cannabinoid Drugs
Table of Contents
| 1. | Cannabinoid Pipeline Report Introduction |
| 2. | Cannabinoid Pipeline Report Executive Summary |
| 3. | Cannabinoid Pipeline: Overview |
| 4. | Analytical Perspective In-depth Commercial Assessment |
| 5. | Cannabinoid Clinical Trial Therapeutics |
| 6. | Cannabinoid Pipeline: Late-Stage Products (Pre-registration) |
| 7. | Cannabinoid Pipeline: Late-Stage Products (Phase III) |
| 8. | Cannabinoid Pipeline: Mid-Stage Products (Phase II) |
| 9. | Cannabinoid Pipeline: Early-Stage Products (Phase I) |
| 10. | Cannabinoid Pipeline Therapeutics Assessment |
| 11. | Inactive Products in the Cannabinoid Pipeline |
| 12. | Company-University Collaborations (Licensing/Partnering) Analysis |
| 13. | Key Companies |
| 14. | Key Products in the Cannabinoid Pipeline |
| 15. | Unmet Needs |
| 16. | Market Drivers and Barriers |
| 17. | Future Perspectives and Conclusion |
| 18. | Analyst Views |
| 19. | Appendix |
For further information on the cannabinoid pipeline therapeutics, reach out @ Cannabinoid Therapeutics
Related Reports
Cannabinoid Agonist Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key cannabinoid agonist companies, including Apurano Pharmaceuticals, IGC Pharma, Inc., Artelo Biosciences, Inc., NeuroTherapia, Oxford Cannabinoid Technologies Holdings, SciSparc, Mira Pharmaceuticals, InMed Pharmaceuticals, Corbus Pharmaceuticals, among others.
Cannabinoid Receptor CB1 Inverse Agonists Pipeline
Cannabinoid Receptor CB1 Inverse Agonists Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key cannabinoid receptor CB1 inverse agonists companies, including Corbus Pharmaceuticals, Inversago Pharma, Goldfinch Bio, Stero Biotechs, Beckley Canopy Therapeutics, Artelo Biosciences, among others.
Cannabinoid Receptor Type 1 (CB1) Antagonist Pipeline
Cannabinoid Receptor Type 1 (CB1) Antagonist Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key cannabinoid receptor type 1 (CB1) antagonist companies, including Skye Biologics Holdings, Aelis Farma, among others.
Alzheimer's Disease Market Insights, Epidemiology, and Market Forecast – 2034 report delivers an in-depth understanding of the disease, historical and forecasted epidemiology, as well as the market trends, market drivers, market barriers, and key Alzheimer's disease companies, including AB Science, Alzheon Inc., AriBio Co., Ltd., AgeneBio, Inc., Anavex Life Sciences Corp., Annovis Bio, Inc., Cerecin, BioVie, Cassava Sciences, Novo Nordisk, Eli Lilly, Neurim Pharmaceuticals, Suven Life Sciences, Bristol Myers Squibb, Karuna Therapeutics, T3D Therapeutics, Inc., Lexeo Therapeutics, Axsome Therapeutics, Inc., Araclon Biotech S.L., Eisai Co., Ltd., TauRx Therapeutics, TrueBinding, Inc., AC Immune SA, Johnson & Johnson, Longeveron Inc., Vaccinex Inc., IGC Pharma LLC, among others.
Alzheimer's Disease Pipeline Insight – 2025 report provides comprehensive insights about the pipeline landscape, pipeline drug profiles, including clinical and non-clinical stage products, and the key Alzheimer's Disease companies, including Biogen, AZTherapies, Cerecin, Neurotrope, Synaptogenix, INmune Bio, Cassava Sciences, EIP Pharma, Neuraly, AB Science, Cortexyme, Anavex Life Sciences, Athira Pharma, Time Therapeutics, Denali Therapeutics Inc., Alector Inc., Lexeo Therapeutics, TrueBinding, Inc., Vaccinex Inc., Annovis Bio Inc., Eisai Inc., Hoffmann-La Roche, Ionis Pharmaceuticals, Inc., Otsuka Pharmaceutical Co., Ltd., Cognition Therapeutics, Merck Sharp & Dohme LLC, ImmunoBrain Checkpoint, AbbVie, AriBio Co., Ltd., Oryzon Genomics S.A., Eli Lilly and Company, Neurokine Therapeutics, Excelsior, Seelos Therapeutics, Inc., Janssen Research & Development, LLC, Shanghai Hengrui Pharmaceutical Co., Ltd., reMYND, Alzinova AB, VTBIO Co. LTD, BioVie Inc., Prothena Corporation plc, Coya Therapeutics, Inc., among others.
DelveInsight’s Pharma Competitive Intelligence Service: Through its CI solutions, DelveInsight provides its clients with real-time and actionable intelligence on their competitors and markets of interest to keep them stay ahead of the competition by providing insights into the latest therapeutic area-specific/indication-specific market trends, in emerging drugs, and competitive strategies. These services are tailored to the specific needs of each client and are delivered through a combination of reports, dashboards, and interactive presentations, enabling clients to make informed decisions, mitigate risks, and identify opportunities for growth and expansion.
Other Business Pharmaceutical Consulting Services
Healthcare Conference Coverage
Discover how a mid-pharma client gained a level of confidence in their soon-to-be partner for manufacturing their therapeutics by downloading our Due Diligence Case Study
About DelveInsight
DelveInsight is a leading Business Consultant and Market Research firm focused exclusively on life sciences.
Connect with us at LinkedIn

Contact Us Shruti Thakur info@delveinsight.com +14699457679 www.delveinsight.com
Legal Disclaimer:
EIN Presswire provides this news content "as is" without warranty of any kind. We do not accept any responsibility or liability for the accuracy, content, images, videos, licenses, completeness, legality, or reliability of the information contained in this article. If you have any complaints or copyright issues related to this article, kindly contact the author above.
